Biochemistry Module
Greening Across the Chemistry Curriculum English | Versión en Español  | Versão em Português (Brasil)
| Versão em Português (Brasil) 
A Green Chemistry Module
Suggested Use: A biochemistry course during a discussion of enzyme mechanisms and kinetics, or oxidative phosphorylation.
A New Chemical Family of Insecticides Exemplified by CONFIRM Selective Caterpillar Control Agent and the Related Selective Control Agents MACH 2 and INTREPID
offers a "Green" Alternative to Some of the More Conventionally Used Insecticides
Joan Wasilewski, Chemistry Department, University of Scranton
joan.wasilewski@scranton.edu
Introduction
Insecticides are used in the farming industry in order to prevent widespread plant destruction. There is a variety of classes of insecticides used. Those discussed below include the organophosphates and carbamates, which are widely used chemicals whose primary effect is the inhibition of an enzyme involved in regulation of nerve transmission; and rotenone, a chemical whose effect involves inhibition of an enzyme found in the electron transport chain. Although these classes of chemicals are effective against insects, they may exhibit toxic effects on other organisms.
A new family of insecticides developed by Rohm and Haas is not only effective against insects, but offers a higher level of selectivity than is often found with other insecticides. Rohm and Haas received a Presidential Green Chemistry Award for their development of these insecticides (CONFIRM, INTREPID, and MACH 2). (1) One of the insecticides, CONFIRM, has been classified by the United States Environmental Protection Agency as a reduced risk pesticide.
In this module, the mode of action for each of these classes of insecticides will be discussed.
Background
Pesticides are materials used to prevent or decrease the proliferation of pests, e.g.. insects, microbes, rodents, algae, fish, plants, etc. A more extensive list of targets of pesticides can be seen on the web site of the U. S. Environmental Protection Agency at https://www.epa.gov/opp00001/whatis.htm. The most common uses of insecticides are in farming and gardening. Insecticides are also found in products designed for tick and flea protection and for general insect control.
Areas of concern when working with pesticides include environmental contamination and nonselectivity. Contamination of the water supply can have toxic effects on plants and wildlife in the area of administration. Contamination of the food being produced can have widespread effects, as well. It is important to keep in mind that pesticides work by suppressing some important biological mechanism of the target organism. However, since the series of metabolic reaction pathways that occur in cells is quite similar from organism to organism, pesticides may pose threat to nontarget organisms, as well. Whether these nontarget organisms are humans, wildlife, or plant life; the use of pesticides may pose a threat to the environment that should be considered carefully. The choice of pesticide is an important one. A pesticide which is highly selective toward a given organism will pose a lower risk to the environment.
Examples of Pesticides
*organophosphates
One of the most widely used classes of insecticides are organophosphates. They account for approximately one half of the insecticides used in the United States (2). Organophosphates irreversibly inhibit the important enzyme acetlycholinesterase which is important in the regulation of levels of acetylcholine, a neurotransmitter. Some can be highly toxic and are chemically related to nerve gases. Others are considerably less toxic.
* carbamates
Carbamates make up another class of pesticides. They inhibit acetlycholinesterase, as do the organophosphates, but they do so reversibly.
*a botanical pesticide—rotenone
Rotenone is derived from the roots, seeds, and leaves of various plants and is referred to as a botanical pesticide. It is a nonspecific pesticide used for insect control on both plants and pets, as well control of fish populations. (3) Rotenone is an inhibitor of an enzyme in one of the complexes involved in the oxidation of NADH in the electron transport chain.
*molting accelerators--diacylhydrazines (GREEN CHEMISTRY)
The diacylhydrazines are agonists of the insect steroidal hormone 20-hydroxyecdysone (an ecdysteroid). This steroidal hormone is proposed to be involved in regulation of genetic expression of the insect genome. The effect is disruption of the molting process of target insects, resulting in death.
Modes of Action
Acetylcholinesterase Inhibition
function of acetylcholine
Both organophosphates and carbamates act by inhibiting the enzyme acetylcholinesterase which catalyzes the hydrolysis of acetylcholine to choline and acetate. Acetylcholine is a neurotransmitter which is synthesized by the transmitting, or presynaptic, cell. It is stored in synaptic vesicles until an action potential causes a depolarization of the presynaptic cell's plasma membrane. This results in a Ca2+ influx followed by fusion of the synaptic vesicles with the plasma membrane. Acetylcholine is released into the synaptic cleft and diffuses to the receiving, or postsynaptic, cell. Here, the acetylcholine binds to a its receptor, which can act as a cation channel. The receptor undergoes a conformational change upon binding the acetylcholine. This change in three-dimensional structure results in an influx of Na+ which depolarizes the plasma membrane in the postsynaptic cell. This initiates an action potential.
It is necessary for acetylcholine to be removed from the synaptic cleft before the synapse is able to respond to another signal. This requires the action of acetylcholinesterase, which catalyzes the reaction below:
acetylcholine + H2O ----------> choline + acetate
The choline is taken up by presynaptic cells and used in the synthesis of more acetylcholine. The acetate is transported to other tissues and metabolized.
acetlycholinesterase catalysis
Enzymes are biological catalysts. As such, they increase the rate of a chemical reaction without being consumed. Most enzymes are highly specific with respect to the reaction being catalyzed. The reaction catalyzed by acetlycholinesterase is shown below:

The diagram below shows the binding of acetylcholine to the active site of acetlycholinesterase and the catalysis at the active site:
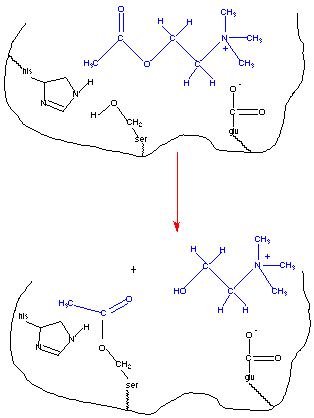
This figure depicts the binding of acetylcholine to the active site of acetylcholinesterase. Amino acid side chains play important roles in forming the enzyme-substrate complex and more directly in the catalysis process. The first step in any enzyme catalyzed reaction is the binding of the substrate to the active site of the enzyme. This is facilitated by interactions between the substrate and amino-acyl side chains on the enzyme. Often these are noncovalent interactions and include electrostatic attractions, hydrogen bonding, and van der Waals attractions. Negatively charged residues can interact favorably with the positively charged amino group. The histidine residue is near the serine residue and may stabilize the conformation of the active site via hydrogen bonding. In fact the activity of the enzyme is pH dependent with maximal activity at a pH value consistent with the pKa value of a histidine residue (4). In addition to amino acid residues involved in formation of the enzyme-substrate complex, there are those involved more directly in the catalysis. Note the serine residue located at the active site. Its nucleophilic hydroxyl group reacts with the acyl carbon on acetylcholine. The serine is acetylated and choline is the leaving group.
Enzymes, as catalysts, are not consumed during the course of the reaction; and must be regenerated before the reaction is complete. In the figure below, the acetylated serine residue must be hydrolyzed before the reaction is complete and the enzyme is capable of catalyzing a new reaction.

enzyme inhibition, organophosphates, and carbamates
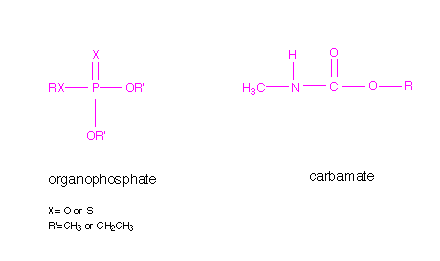
Inhibitors are substances that interact with an enzyme and result in a decrease in activity. Inhibitors are referred to as reversible or irreversible, depending of the lifetime of the enzyme/inhibitor complex. Organophosphates are irreversible inhibitors, reacting with the serine residue at the active site and remaining attached. The reaction with carbamates is similar, but the complex is not as long-lived. The general structures of organophosphate and carbamate insecticides are shown below.
The nucleophilic hydroxyl group of the serine residue located at the active site of the acetylcholinesterase reacts with the phosphorus of the organophosphate resulting in a covalently bound organophosphate. Likewise, this same nucleophilic hydroxyl group reacts with the carbonyl carbon resulting with a covalently bound carbamate, similar to the reaction with acetylcholine. The result is inactivation of the enzyme since the hydroxyl group is no longer available to attack the acetylcholine substrate. In the case of irreversible inhibitors, this is referred to as suicide inhibition. A diagram of the organophosphate and carbamate covalently linked to the serine residue at the active site of acetylcholinesterase is shown below.
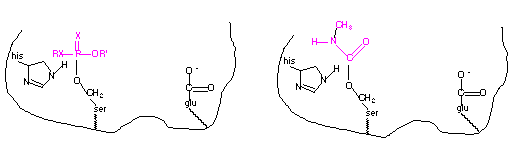
The organophosphates and carbamates can also inhibit the acetylcholinesterase in nontarget organisms, including mammals. Their acute toxicity is due to their effect of the nervous system of animals.
Inhibition of the Electron Transport Chain
the electron transport chain and oxidative phosphorylation
In chemotropic organisms, energy is derived from the oxidation of organic molecules. Fuels such as carbohydrates, fats, and proteins undergo a series of metabolic reactions and are often oxidized to carbon dioxide. Much of the energy liberated in reaction pathways such as the TCA cycle and b-oxidation of fatty acids is in the form of the reduced cofactors nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2). Adenosine triphosphate (ATP), the energy currency of cells, is produced in the mitochondria in a series of reactions initiating with the oxidation of NADH or FADH2. The reactions are collectively referred to as oxidative phosphorylation and include a series of oxidation/reduction reactions in the electron transport chain. The energy produced during the exergonic oxidation of NADH is used to drive the energetically unfavorable phosphorylation of adenosine diphosphate yielding ATP. ATP is used to drive many energetically unfavorable reactions in the body. The hydrolysis of the terminal phosphoanhydride bond is highly exergonic. This energy may be used for muscle contraction and in maintenance of concentration gradients (and numerous other examples).
Below is a diagram of the of electron transport which occurs during the oxidation of NADH.
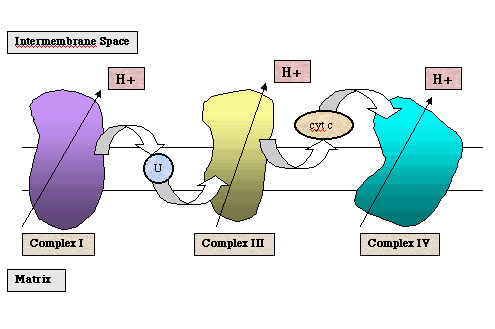
Oxidative phosphorylation takes place in the mitochondria, a double membrane organelle. The oxidation of NADH or FADH2 takes place within protein complexes of the electron transport chain. These complexes contain electron carriers, such as flavins, iron-sulfur centers, hemes (which contain iron), and copper. They are embedded within the inner membrane of the mitochondria. The inner membrane separates the chemically distinct mitochondrial matrix and the intermembrane space.
During the oxidation of NADH, electrons enter the electron transport chain at Complex I (also referred to as NADH dehydrogenase or NADH-Q reductase). As the electrons move through Complex I via a series of redox reactions, protons are pumped from the mitochondrial matrix into the intermembrane space. The electrons are transferred to the first shuttle molecule, ubiquinone, designated "U" in the above figure. The ubiquinone is reduced to ubiquinol which diffuses to Complex III. As the electrons move through Complex III (also referred to as cytochrome c reductase), protons are again pumped from the mitochondrial matrix to the intermembrane space. The electrons are transferred to the second shuttle molecule, cytochrome c. The reduced cytochrome c diffuses to Complex IV (also referred to as cytochrome oxidase). Again protons are pumped from the mitochondrial matrix to the intermembrane space. The ultimate electron acceptor is molecular oxygen which is reduced to water.
The movement of protons from the mitochondrial matrix to the intermembrane space generates an electrochemical potential across the inner mitochondrial membrane. The proton gradient that is generated across the inner membrane is required by the enzyme ATP synthase, which also contains a proton pump. Following the phosphorylation of ADP, the enzyme must release the ATP. This release is dependent on the presence of a proton gradient. This process is referred to as the chemiosmotic theory.
The series of reactions in the electron transport chain yields a proton gradient which is required for the release of ATP from ATP synthase. These processes are coupled. Therefore, if an inhibitor of the electron transport chain were present, it would negatively affect the production of ATP.
rotenone
Rotenone, a botanical pesticide, is an inhibitor of one of the enzymes of Complex I of the electron transport chain. In the presence of this insecticide, electrons from NADH cannot enter the electron transport chain, resulting in the an inability to produce ATP from the oxidation of NADH. The enzyme inhibited by rotenone is NADH dehydrogenase. Rotenone affects cellular respiration and may also affect muscle coordination. The chemical structure of rotenone is shown below.
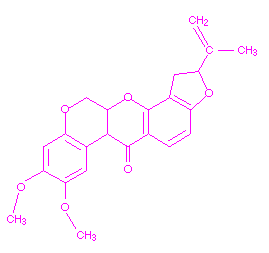
Molting Accelerators
Molting accelerators, including tebufenoxide and halofenozide, are a relatively new class of insecticides which are highly specific for Lepidoptera. These diacylhydrazines are agonists of the insect steroidal hormone 20-hydroxyecdysone (an ecdysteroid) which is required for the molting process. The effect of this class of insecticides is disruption of the molting process of target insects, resulting in destruction. As insect growth regulators, they are more selective than the organophosphates, carbamates, and rotenoids. However, there are few detrimental effects on nontarget organisms.
ecdysone and ecdysone receptors
The steroid 20-hydroxyecdysone is a developmental hormone found in arthropods. The molting process is regulated by varying the concentrations of 20-hydroxyecdysone in the larva (5). Increased levels of the hormone result in initiation of molting. As larva enters the molting stage, they stop feeding and molting fluid containing various proteolytic enzymes required for molting enters the ecdysial space. At this point, epidermal cells enter a phase of increased protein synthesis (gene expression) and a new cuticle is produced. A decrease in the concentration of 20-hydroxyecdysone triggers a subsequent phase which includes activation of enzymes in the molting fluid, digestion of the procuticle, and resorbtion of the molting fluid. Following a further decrease in the concentration of 20-hydroxyecdysone, other hormones are released which are required for completion of the molting process. Feeding then resumes.
Proper growth and development in insects is dependent on the concentration of 20-hydroxyecdysone at various stages of differentiation. This steroidal hormone is proposed to be involved in regulation of genetic expression of the insect genome.
Hormones typically interact with specific receptors prior to initiation of a physiologic effect. The ecdysteroid binds to an ecdysteroid receptor protein resulting in regulation of classes of "early" and "late" genes. (There are numerous examples of this type of temporal regulation in biochemical systems. Some of the simplest are found in a variety of viral systems.) Early genes are activated by the hormone/receptor complex. Protein products of the early genes function to repress transcription of the early genes while at the same time activating transcription of the late genes. Some of the products of the early genes are transcription factors. Transcription factors are proteins that can bind to short nucleotide sequences, resulting in the initiation or regulation of transcription.
diacylhydrazines--"green" insecticides
Some diacylhydrazines can mimic the insect steroidal hormone 20-hydroxyecdysone. The chemical structures of 20-hydroxyecdysone and the diacylhydrazines tebufenozide and halofenozide are shown here.
These insecticides are capable of binding to the ecdysteroid receptor proteins. While gene expression that is dependent on the presence of 20-hydroxyecdysone occurs in the presence of diacylhydrazines; gene expression that is dependent on the absence of 20-hydroxyecdysone is prevented (5). The toxic effect of the diacylhydrazines is due not only to the fact that they mimic the 20-hydroxyecdysone, but also because their levels do not decrease.
Rohm and Haas uses tebufenocide in the insecticide CONFIRM. This diacylhydrazine is selective for lepidopteran insects. It is a highly selective pesticide, with minimal toxicity to nontarget organisms, even nonlepidopteran insects. In the presence of the tebufenocide, the molting process begins. Whereas the 20-hydroxyecdysone levels normally decrease during the molting process, the tebufenocide is more long-lasting. The molting process is halted and feeding does not resume, resulting in death. Rohm and Haas employs halofenozide, another diacylhydrazine, in the insecticide MACH2. Its mode of action is similar to tebufenocide. However, its target is different. This insecticide is used for lawn grubs.
As indicated previously, Rohm and Haas received a Presidential Green Chemistry Award for their work with these insecticides and CONFIRM has been classified as a reduced risk pesticide by the U.S. E.P.A.
Conclusion
There are a variety of mechanisms by which insecticides can eradicate their target populations. Insecticides allow for an increase in crop production and are therefore important to the farming industry. Some insecticides work by affecting biochemical processes that are common to many types of organisms. Examples addressed here are acetylcholinesterase inhibitors and an inhibitor of an enzyme found in the electron transport chain of oxidative phosphorylation. The potential for undesirable effects on the environment is larger with these types of insecticides than with those that are more target specific, such as the molting accelerators.
Questions
1. What is the chemical reaction catalyzed by acetylcholinesterase?
2. Name two classes of insecticides that affect the activity of acetylcholinesterase. What is their mode of action?
3. Discuss the similarities and differences in the ways in which organophosphates and carbamates work as
insecticides.
4. Why is it important for acetlycholine to be converted to choline and acetate quickly?
5. What are the consequences of inactivation of acetylcholinesterase?
6. Describe the role of acetylcholine in neurotransmission.
7. What is the role of the serine residue at the active site of acetylcholinesterase"
8. How does the oxidation of NADH result in an increase in the amount of ATP available to cells?
9. What is the effect of adding rotenone to a system carrying out oxidative phosphorylation?
11. As electrons from NADH move through the electron transport chain, what is the effect on the pH of the
mitochondrial matrix?
12. Discuss the role of 20-hydroxyecdysone in insect development.
13. How do diacylhydrazines affect insect development?
14. What is the advantage of using an insecticide with a more specific target population?
15. Explain why organophosphate insecticides can be more acutely toxic than diacylhydrazine insecticides to
workers administering them.
References
1. Cann, M. C. and Connelly, M. E., Real World Cases in Green Chemistry,
American Chemical Society: 2000
2. Organophosphate Pesticides in Food.
https://ww.epa.gov/oppsrrd1/op/primer.htm(accessed June 2000)
3. Extoxnet. https://www.pmep.cce.cornell.edu/profiles/extoxnet/pyrethrins-ziram/
rotenone-ext.html (accessed July 2000)
4. https://wcb.ucr.edu/schools/CNAS/entm/tmiller/1/modules/page22.html (accessed August 2000)
5. Dhadialla, T. S., Carlson, G. R., and Le, D. P., New Insecticides with Ecdysteroidal and Juvenile Hormone
Activity. Annu. Rev. Entomol. 1998, 43, 545-569.