Industrial Chemistry Module
Greening Across the Chemistry Curriculum English | Versión en Español  | Versão em Português (Brasil)
| Versão em Português (Brasil) 
A Green Chemistry Module
Suggested Use:An industrial chemistry course in discussions of the petrochemical industry and/or industrial polymerizations or in sections of a polymer course dealing with petrochemical feedstocks.
Petretec – Dupont’s Technology for Polyester Regeneration
Trudy A. Dickneider, Ph.D., Department of Chemistry, University of Scranton
Introduction
Crude Oil – The Beginning of the Story
 The road to any plastic produced today begins with a fossil fuel – oil, natural gas, or coal.Most polymers are produced from petrochemical feedstocks that are the end product of the refining and reforming of crude oil. Crude oil, more properly called petroleum, is a complex mixture of thousands (maybe millions!) of compounds.While most of these compounds are hydrocarbons, some contain oxygen, nitrogen, or sulfur, and there are trace amounts of metals, usually present in large molecules called porphyrins.The scheme below shows the average composition of crude oil along with some representatives of each class of compounds.3
The road to any plastic produced today begins with a fossil fuel – oil, natural gas, or coal.Most polymers are produced from petrochemical feedstocks that are the end product of the refining and reforming of crude oil. Crude oil, more properly called petroleum, is a complex mixture of thousands (maybe millions!) of compounds.While most of these compounds are hydrocarbons, some contain oxygen, nitrogen, or sulfur, and there are trace amounts of metals, usually present in large molecules called porphyrins.The scheme below shows the average composition of crude oil along with some representatives of each class of compounds.3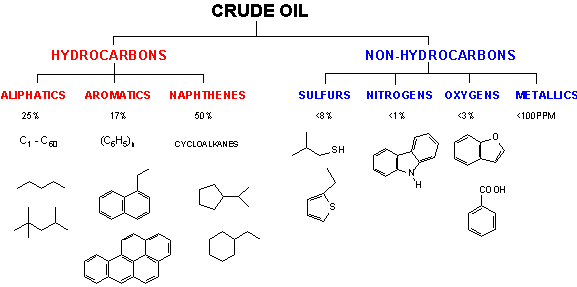

The American Petroleum Institute (API) has developed a universal system for classifying crude oils based on their density.Rather than expressing the density in the traditional terms of weight per unit volume, the API gravity is described in degrees of the API scale.Petroleum characteristics as a function of API gravity are summarized below.
|
API Range
|
Description
|
Viscosity
|
Color
|
Components
|
|
0o – 22.3o
|
HEAVY
|
Extreme
|
Dark
|
Asphalt
|
|
22.3o – 31.3o
|
MEDIUM
|
Moderate
|
brown
|
Gasoline & diesel
|
|
31.3o – 47o
|
LIGHT
|
Fluid
|
Light yellow
|
Condensate/gasoline
|
An average crude oil with an API gravity of 35o would have the following composition:
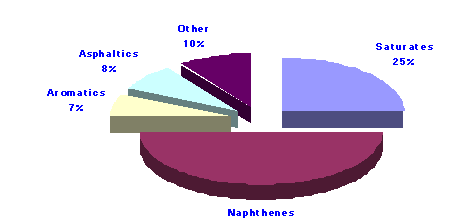
It is estimated that the world reserves of petroleum may total 2 trillion barrels.4 However, the known and proven, in the ground, reserves total some 1034 thousand million barrels.That’s just over 1 billion barrels.While this seems like a huge number, all fossil fuels are non-renewable resources so conservation and wise use of the petroleum produced are imperative.
The products that come to use from crude oil are almost too numerous to mention.Petroluem products are involved in the manufacture of goods used in home and commercial construction, automobiles, fibers for clothing, holiday decorations, food processing and packaging, medical devices, and the synthesis of pharmaceuticals. The list goes on and on as can be seen at https://www.api.org/edu/petprods.htm. This site, sponsored by the American Petroleum Institute, summarizes many petroleum products in everyday life.The route from crude oil to sweaters, CD’s, car bumpers, roofing shingles, etc., is a long one involving a great deal of chemistry called refining and reforming.The products which can be derived from an average barrel of crude oil, which contains 42 gallons, are shown below.5
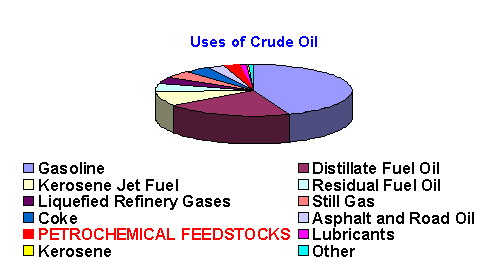
In this module we are most concerned with the petrochemical feedstocks which account for 2.7% (by volume) of every barrel of crude. In general, petrochemical feedstocks can be categorized by source. Shown below are the feedstock chemicals derived from each major fossil fuel source.

Some 40 million barrels of these feedstocks are used daily in the world’s refineries to produce petrochemicals which are then used by other aspects of the chemical industry to produce intermediates for further production or consumer goods.4We can see, then, that crude oil produces two major categories of products – fuels and feedstocks.
While petroleum has been found in some amount on every continent, the major commercial reserves are summarized below. 6
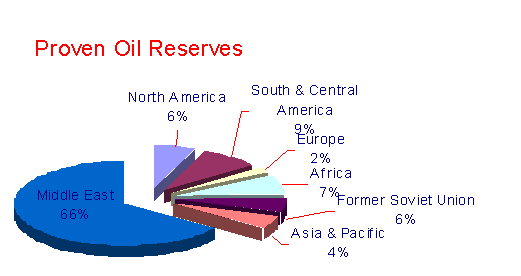
The composition of crude oils from each of these areas differs, therefore, its commercial value is also different.High grade crudes which directly produce large amounts of gasoline have the most commercial value.Those which need considerable reforming to produce significant amounts of gasoline or contain larger than usual amounts of metals such as vanadium (which poisons or shortens the life of the catalysts used in reforming) have the lowest dollar value. The average crude we discussed above with an API Gravity of 35o, after refining would yield the product mixture shown below.3
|
Refinery Product
|
Hydrocarbon Range
|
Percent
|
|
Gasoline
|
||
|
Kerosene
|
||
|
Diesel
|
||
|
Heavy Gas Oil
|
||
|
Lubricating Oil
|
||
|
Residuum
|
While there are direct markets for the fuels (gasoline, kerosene, and diesel) in order to be profitable the other components of the crude oil, especially the gas oil and residuum need to be converted into marketable products. This is the role of reforming.
Producing the Para-xylene – Refining and Reforming
 The dimethylbenzenes, commonly known as the xylenes, are important industrial chemicals. They are used in the manufacture of dyes, and in the production of benzoic acid, phthalic anhydride, and the iso- and terephthalic acids. The dimethyl esters of these acids are used in polymerization reactions producing a large family of polyesters.It is one of these, polyethylene terephthalate that is the subject of this module. The production of polyethylene terephthalate begins with the para isomer of xylene.The three isomeric xylenes can be produced from coal tar distillate, but the major source is crude oil.A mixture of isomers is recovered from the fossil fuel source. It contains 50-60% of meta-xylene and 20-25% each of the ortho and para isomers. The first step in recovering the p-xylene used for polymer production is its separation from the other isomers.As can be seen from the xylene family data shown below, the boiling points of the meta and para isomers are very similar. The difference between their boiling points and that of the ortho isomer allow them to be fractionally distilled from the ortho isomer. When the distillate cools, the para isomer crystallizes allowing it to be separated from the meta isomer by fractional crystallization.
The dimethylbenzenes, commonly known as the xylenes, are important industrial chemicals. They are used in the manufacture of dyes, and in the production of benzoic acid, phthalic anhydride, and the iso- and terephthalic acids. The dimethyl esters of these acids are used in polymerization reactions producing a large family of polyesters.It is one of these, polyethylene terephthalate that is the subject of this module. The production of polyethylene terephthalate begins with the para isomer of xylene.The three isomeric xylenes can be produced from coal tar distillate, but the major source is crude oil.A mixture of isomers is recovered from the fossil fuel source. It contains 50-60% of meta-xylene and 20-25% each of the ortho and para isomers. The first step in recovering the p-xylene used for polymer production is its separation from the other isomers.As can be seen from the xylene family data shown below, the boiling points of the meta and para isomers are very similar. The difference between their boiling points and that of the ortho isomer allow them to be fractionally distilled from the ortho isomer. When the distillate cools, the para isomer crystallizes allowing it to be separated from the meta isomer by fractional crystallization.
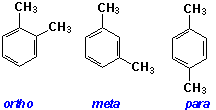
The XYLENES |
ortho
|
meta
|
para
|
|
Boiling point
|
144oC
|
139.3oC
|
137-138oC
|
|
Melting point
|
-25oC
|
-47.4oC
|
13-14oC
|
In 1999 some 8,802 million pounds of para-xylene were produced. 7This was an increase of 5 percent over the previous year and represents nearly 20% growth over the last two years. This make p-xylene one of the fastest growing of the industrial petrochemicals. The total capacity for p-xylene production is 10,080 million pounds so yearly production is running at nearly 90% of capacity to meet industrial demand.Several of the large oil companies are major suppliers of p-xylene, including Mobil, Exxon, and Chevron. The most important producer is Amoco, with its plants in Decatur, Alabama, and Texas City, Texas, supplying nearly half of the total yearly market. In addition, the Amoco plants are the only suppliers to directly produce the purified terephthalic acid needed for polymerization. 8
Although there are other uses for the xylenes in the dye and perfume industry, and p-xylene is used as a solvent and is important in the manufacture of some herbicides, almost all of the p-xylene produced is used in the manufacture of purified terephthalic acid (PTA). The PTA is converted to polyester fibers, resins, and films, and into dimethyl terephthalate (DMT).
The xylenes, along with countless other industrially important petrochemicals are produced from crude oil by the processes of refining and reforming. The umbrella term refining is often used to describe the process of conversion of crude oil into useful products. However, there are at least five distinct processes involved.
|
|
|
|
|
|
|
|
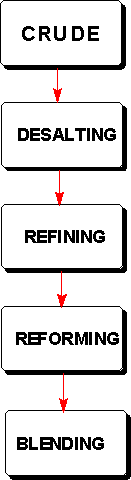 The process really begins in the oil field where the crude oil is recovered from the reservoir in which it is stored by drilling. The reservoir rock is usually several thousand feet underground. The oil is produced from the well, collected and transported by tanker or pipeline to the refinery.
The process really begins in the oil field where the crude oil is recovered from the reservoir in which it is stored by drilling. The reservoir rock is usually several thousand feet underground. The oil is produced from the well, collected and transported by tanker or pipeline to the refinery.
The first step in the treatment of the crude oil is DESALTING in which the crude oil is washed to remove suspended water, salt, dirt, and other non-organic impurities which may be part of the oil or may have contaminated it during production.The desalted oil then enters the REFINING process where it is distilled under both atmospheric pressure and vacuum conditions. This distillation produces some marketable products but many components require conversion in which the composition of the products, their molecular structures, are modified. This is known as REFORMING.
The reformed products then undergo BLENDING, a process which combines some of the straight run distillation products with portions of the converted products to produce gasolines and other products whose compositons have been designed to provide needed commercial properties.These operations are conducted in a refinery, usually a huge complex covering many acres, containing distillation towers, processing and storage tanks, and large-scale reactors all connected by miles of pipelines. While desalting and blending are straightforward processes, the refining and reforming, the place where the chemistry happens, need further examination. A detailed diagram of a refinery can be seen athttps://www.chevron.com/explore/science/refinery/chart.html.
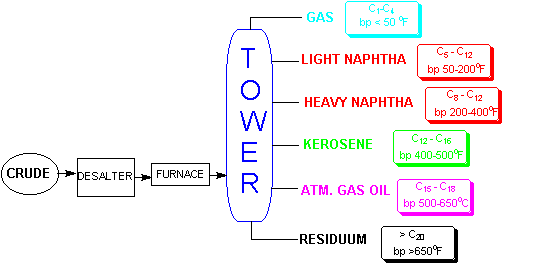
A refining tower is a huge (sometimes > 100 feet tall) distillation column. The crude oil is continuously feed into the tower and the distilled products are continuously removed. In this way a refinery can distill thousands of barrels of oil a day. The oil, which has an average boiling point of around 420oC is heated in a furnace which vaporizes most of the oil and the gases and hot liquids are fed into the base of the tower.9 The interior of the tower consists of a series of plates, known as bubble plates, on which the gases condense and then are revaporized to condense on the next higher plate, where they are again vaporized to condense farther up the tower. The components with the lowest boiling point exit the top of the tower, and the materials with the highest boiling point remain at the bottom of the tower. In this way the components of the petroleum are separated by boiling point according to their weight. These products are known as straight run products. Certainly, the gasoline produced has a market at this point, but since usually less than 30% of an average crude oil will distill straight run gasoline, conversion of the remainder, especially the residuum, is needed to maximize the value of the crude oil. A schematic of the refining process is shown below.
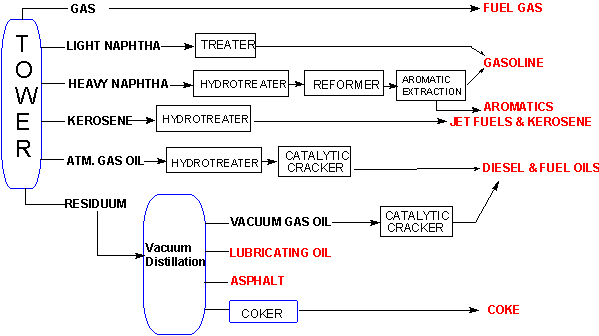
Reforming involves the chemical transformation of the products from the distillation. The reactions which take place change the size and structure of the molecules in the distillation fractions, producing usable materials from the residuum and converting other products to ones with a higher commercial value.9,10These reactions involve the actions of heat and pressure, often in the presence of a catalyst and are often designated as PROCESSING REACTIONS.The schematic below shows the flow of straight run products from the refining tower through the processes of reforming to finished products.
Reactions to combine molecules include alkylation and polymerization reactions.11 In an alkylation reaction alkenes are bonded to an alkane or an aromatic compound.These reactions also allow production of brached alkanes by combination of a straight chain alkene with an isoalkane. Both types of reactions are shown below.
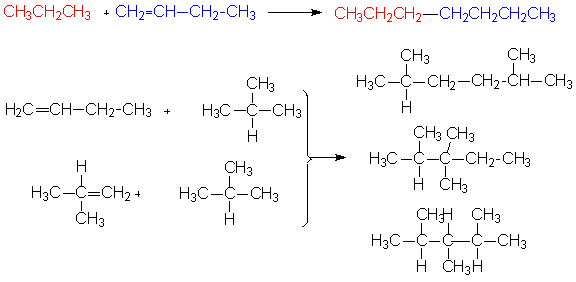
Molecules can also be combined through polymerization reactions in which alkenes are linked together under the action of heat, pressure, and a catalyst. A sample reaction is shown below.
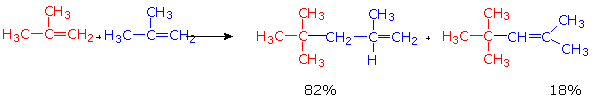
Often it is necessary in conversion reactions to change the molecular structure, not by adding alkyl fragments or breaking chains, but rather by rearranging the original structure. This can be accomplished by reforming reactions. Catalytic reforming and isomerization reactions are used to improve the octane number of distillation products and to produce aromatic compounds for chemical manufacturing.In these processes, naphtha from the refining process is converted to a mixture of compounds known as reformate. The C6 to C8 compounds in the naphtha are converted to alkanes, cycloalkanes, and aromatics. The composition of the aromatics is of particular importance for the subject of this module. The aromatics are a mix of benzene (16%), toluene (47%), and the xylenes (37%).Examples of these reactions are shown below.11
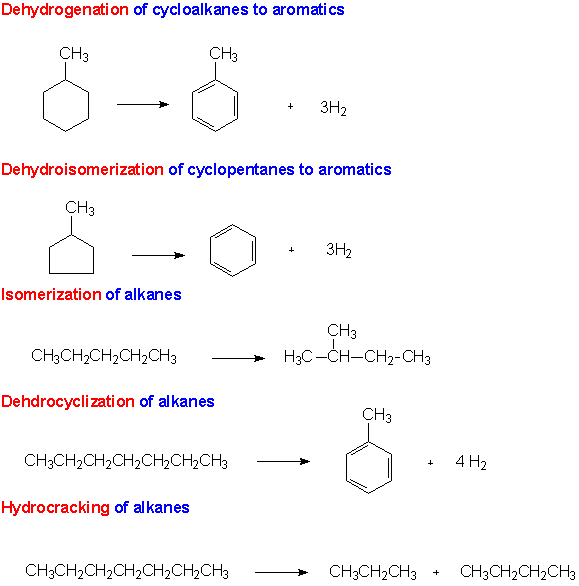
The operations conducted in a refinery also include many mechanical techniques and processes involving improving the physical characteristics of the refined and reformed products. These include dewaxing, and extracting. Some of these are shown in an excellent refinery diagram on the Amoco web site (https//www.Amoco.com/resource_pool/design/refinery_flow.jpeg)
The process of refining and reforming crude oil produces the fuels for heating and transportation needs, lubricating oils, greases, and asphalts for mechanical devices and road construction as well as the petrochemical feedstocks for chemical manufacturing.One of the most important areas of chemical manufacturing involves polymerization reactions.
Polymerization – Reactions for Linking the Pieces
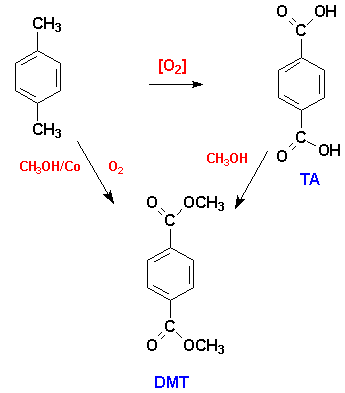
The DMT produced from the one step reaction must undergo a five column distillation procedure to yield material pure enough to be used in polymerization reactions.
Every year C&E News surveys the quantities of chemicals produced and lists the Top 50 Chemicals. Both TA and DMT are listed and they are the only chemicals in the top 50 that are derived from p-xylene.
The PET is produced by means of a polymerization reaction of these two monomers – the ethylene glycol and the dimethyl terephthalate. Polymers are compounds composed of repeating units. The monomeric units are linked together in a polymerization reaction to form the oligomer, consisting of many units. The polymer industry produces over 60 billion pounds of polymers per year.The industry consumes about 130 billion pounds of chemical feedstocks per year.11 This accounts for nearly half of the organic compounds produced each year in the United States. Furthermore, nearly half of the chemists employed in the chemical industries work with polymers either in synthesis or manufacturing.
There are basically two types of polymerization reactions. In chain growth polymerization the monomers continue to add to a growing chain once the reaction has begun. This produces polymers of high molecular weight and involves reactions with ionic or free radical intermediates. The other process, known as step growth polymerization involves a condensation reaction in which the two functional groups react with each other to eliminate a small neutral molecule, usually water. This polymerization can be controlled to limit the length of the chain and to give a low molecular weight polymer.
Polyethylene terephthalate (PET) is a polyester polymer. Polyesters can be synthesized two ways. The first method is a direct reaction of a diacid with a diol. To produce PET, terephthalic acid is reacted with ethylene glycol as shown below.
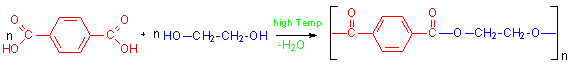
This reaction is a typical Fisher type esterification in which an acid is reacted with an alcohol and follows the usual mechanism for that reaction. The fact that each molecule is difunctional produces a polymer by the reaction.
The other synthesis of PET involves an ester interchange of a diester and a diol.This is a transesterification reaction in which one ester is transformed into another.The synthesis of PET by this method reacts dimethylterephthalate with ethylene glycol as below.
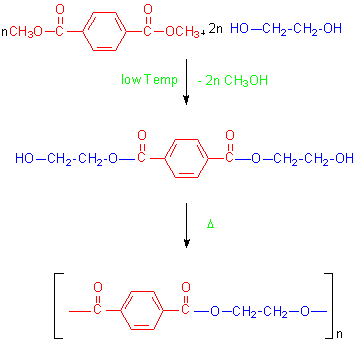
The original synthesis of PET was performed by Whinfield and Dixon.11 They used a transesterfication of DMT and glycol in a 1 : 2.4 ratio, distilling methanol out of the reaction mixture as the synthesis progressed.Their polymerization was conducted at 200-290oC in the presence of an SbO3 catalyst.Later techniques employed in the polymer industry used a step growth polymerization of terephthalic acid with an excess of ethylene glycol at 250oC at a reaction pressure of 60psi.This formed a polymer of from 1 to 6 repeating units. During the 1970’s syntheses of PET used three times as much DMT as TA. By the 1980’s the amounts of DMT and TA being used were nearly equal. In the United States today the ratio of TA to DMT used in polyester synthesis is TA 46 : DMT 54.
The initial problem with using TA was the purity of the acid.The esterification to DMT allowed separation of a pure product.The use of TA increased as techniques became available for producing pure terephthalic acid, known as PTA. The PTA is 99% pure. It is produced from the oxidation of p-xylene in the presence of cobalt and manganese salts of heavy metal bromides. 10,11
As noted earlier, Amoco is the major supplier of PTA. The Amoco process for the production of PTA crystallizes the crude TA (90% yield of 99.6% pure TA). When the acetic acid by product and the unreacted p-xylene are removed by evaporation, the TA is further purified by washing in hot water.The major impurity remaining is p-formylbenzoic acid which is hydrogenated to p-methoxybenzoic acid. The TA can then be separated by fractional crystallization which yields PTA which is 99.9% pure terephthalic acid.The uses of these two monomers, TA and DMT, are shown below. 11
|
Percentage of Total
|
|
|
Polyester fiber
|
|
|
Exports
|
|
|
Polyester resin
|
|
|
Polyester film
|
|
|
Miscellaneous
|
The Brown Side of the Story – Uses and Abuses of PET
Focusing on the recycling problems of PET, Dupont developed four goals, according to Mike Harnagel, the vice president and general manager of Dupont Films.13 These goals were to:
- avoid landfilling polyester waste materials
- increase public awareness of the greenness of polyester materials
- reduce overall consumption of oil derivatives
- retain the chemical value of polyester
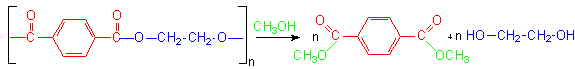
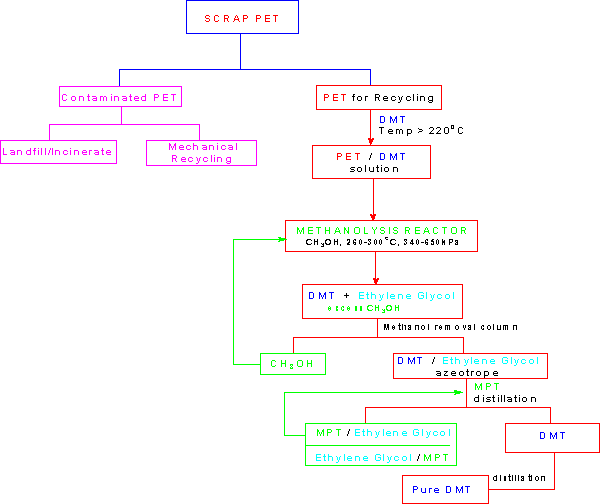
The depolymerization reaction that is the heart of the Petretec technology produces DMT and ethylene glycol, the molecules from which the PET was formed, not polyester flake, the product of mechanical recycling. The tremendous advantage of this is that these reproduced monomers are identical to those used as raw materials for the polymerization reaction. Therefore, there are no limits on the uses of the PET made from them. This results in a reduction of the dependence on petrochemicals for production. The brown side of PET is largely overcome by the green Petretec process.Dupont operates the Petretec process at their Cape Fear facility in North Carolina.The plant has a reprocessing capacity of 100 million pounds per year.2
Study Questions:
- What is the molecular composition range for each of the categories listed in the “Uses of Crude Oil”? What is meant by terms such as still gas, residual fuel oil, coke, etc.?
- What is octane number? What types of compounds have the highest octane numbers, what compound class has the lowest octane numbers? Why is this so?
- Draw a reasonable mechanism for the alkylation reaction that is part of the conversion processes involved in reforming of petroleum. What factors would determine whether the reaction involves free radicals or ionic intermediates?
- Outline a mechanism for the reactions between TA and ethylene glycol and DMT and ethylene glycol to produce PET.
- What would be the economic advantages of using TA (as PTA) in the production of PET, rather than DMT?
- Outline a synthesis of poly(butylenes)terephthalate from both TA and DMT. What are the major uses of PBT?
- Outline a reasonable mechanism for the transesterification reaction that is the heart of the Petretec process.
- What is an azeotrope? What is the boiling point range of an azeotrope ?
Suggestions for Further Study
References
- Morse, P.M., PET Producers Face Rough Transition Maket. Chem. Eng. News, July 22, 1998, pp 33-35.
- Cann, M.C., Connelly, M.E., “Dupont Petretec Polyester Regeneration Technology” in Real-World Cases in Green Chemistry. American Chemical Society (2000).
- Hunt, J. Petroleum Geochemistry and Geology, 2nd Edition, W. H. Freeman and Company (1996) in Chapter 3, Petroelum and its Products.
- Facts and Figures on Oil, American Petroleum Institute, www.api.org/faqs, accessed August 2000.
- What a Barrel of Oil Makes. American Petroleum Institute, www.api.org/edu/factsoil.htm#barrel, accessed August 2000.
- Statistical Review of World Energy, British Petroleum Company, www.bp.com/world energy/, accessed July 2000.
- Facts & Figures for the Chemical Industry, Chem. Eng. News, June 26, 2000.
- Chemical Profile of p-xylene. Fobchemicals.com at www.chemexpo.com/news/PROFILE980515.cfm, accessed July 2000.
- Kent, James A., ed., Riegel’s Handbook of Industrial Chemistry, 8th Edition. Van Nostrand Reinhold Company (1983).
- Austin, George T. Shreve’s Chemical Process Industries, 5th Edition. McGraw-Hill Book Company (1984).
- Chenier, Philip J., Survey of Industrial Chemistry, 2nd Revised Edition. VCH Publishers (1992).
- PET Facts, National Association for PET Container Resources. www.napcor.com/toolbox/funfacts.html, accessed July 2000.
- “It Starts with a Little Imagination”, Dupont Magazine, November/December 1996 www.Dupont.com/corp/products/dupontmag/novdec96/petretec.html, accessed July 2000.
- The Petretec Process of Dupont was a nominee for a 1997 Presidential Green Chemistry Challenge Award. More information about the program is available at the program web site at www.epa.gov/greenchemistry, accessed July 2000.
- Michel, R. Methanolysis of PET. ARC’96 Technol. Spark Recycl. Conf. Proc., 3rd, 349-356. Walling, J., Walling, R. L., eds., Society of Plastics Engineers, Brookfield, CT (1996).
- Michel, R., Jones, P., Everhart, D.. Dupont Polyester Regeneration Technology, a proposal submitted to the Presidential Green Chemistry Challenge Awards Program, 1997.
- Michel, R. E., Recovery of Methyl Esters of Aromatic Acids and Glycols from Thermoplastic Polyester Scrap Using Methanol Vapor. Eur. Patent 484,963, May 13, 1992.
- Hepner, R.R., Michel, R.E.. Process for the Separation of Glycols from Dimethyl Terephtahlate. U.S. Patent 5,391,263, Feb. 21, 1995.
- Michel, R.E., Recovery of Dimethyl Terephthalate from Polymer Wastes. U.S. Patent 5,504,122, April 2, 1996.