Polymer Chemistry Module
Greening Across the Chemistry Curriculum English | Versión en Español  | Versão em Português (Brasil)
| Versão em Português (Brasil) 
A Green Chemistry Module
Suggested Use: A polymer chemistry course after the basic concepts of radical polymerization, step growth polymerization, and the effects of crosslinking on polymer properties have been presented.
Thermal Polyaspartate as a Biodegradable Alternative to Polyacrylate and Other Currently Used Water Soluble Polymers
Donna Narsavage-Heald, Chemistry Department, University of Scranton
Introduction
Scale build up is a problem in industrial water handling processes because it results in reduced water flow though pipes, reduced heat transfer in boilers and condensers, and pump failures. 1, 2, 3, 4 Scale consists of insoluble inorganic compounds such as calcium carbonate, calcium sulfate, and barium sulfate.
Antiscalants are substances that either prevent scale formation entirely or permit the scale to be deposited in such a way that it is easily removed by the fluid flowing along the pipe or heat transfer surface. Antiscalants act by forming complexes with the cations present in water to prevent formation of the insoluble inorganic solids.
The polyanion, polyacrylate (PAC), is one of the most common scale inhibitors (Figure 1).
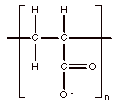
Figure 1. Polyacrylate
Polymers with bound positive or negative charges are referred to as polyelectrolytes, macroions, or polyions. Polyelectrolytes can be polyanions or polycations and are generally water soluble polymers if their structure is linear. Water interacts strongly with polyanions, such as PAC, via hydrogen bonding to the anionic groups (such as the carboxylate oxygens in PAC) aiding in the dissolution of the polymer.
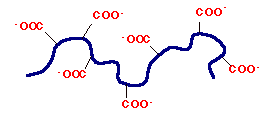
Figure 2. Polyanion
Polyacrylate (PAC)
The synthesis of PAC begins with the free radical polymerization of acrylic acid to produce polyacrylic acid. Polyacrylic acid can then be converted into the polyanion, PAC, by reaction with a base such as sodium hydroxide.
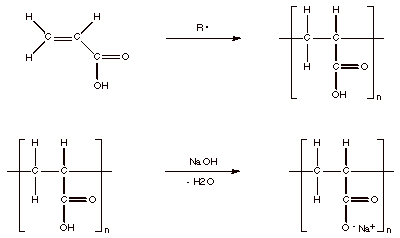
Scheme 1. Synthesis of Polyacrylic Acid and Conversion to Polyacrylate
PAC can function as both an antiscalant and a dispersant. Polymeric antiscalants are generally low molecular weight polymers, whereas polymeric dispersants consist of higher molecular weight fractions. Dispersants do not stop the formation of scale, but instead are able to keep the scale particles suspended in the bulk fluid by imparting a negative charge to the particles. These negatively charged repel one another and prevent aggregation (and precipitation) into larger particles of scale. PAC comprises about 5% of many laundry detergent formulations because of its dispersant properties.4, 5
PAC can be crosslinked by using a multifunctional vinyl monomer. Typically, trimethylolpropanetriacrylate (Figure 3) is used in commercial processes as a crosslinking agent.6
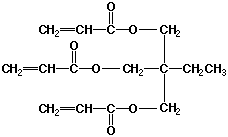
Figure 3. Crosslinking Agent, Trimethylolpropanetriacrylate
A crosslinked form of the sodium salt of polyacrylic acid is used as a superabsorbant material in diapers and other personal hygiene products. Crosslinked PAC has a great affinity for water, but because of the crosslinks it is unable to dissolve in water and will instead swell in aqueous solution. Water still binds strongly to the anionic sites on the exterior and interior surfaces of the molecule. Although the crosslinked polymer does not dissolve, water entering and binding to the interior of the polymer structure is what causes the polymer to swell. Figure 4 compares a crosslinked polymer in the absence of solvent with a crosslinked polymer that is swollen in a compatible solvent. Because of the presence of the charged groups on the polymer chain of a polyelectrolyte, the polymer will be highly expanded in aqueous solution.
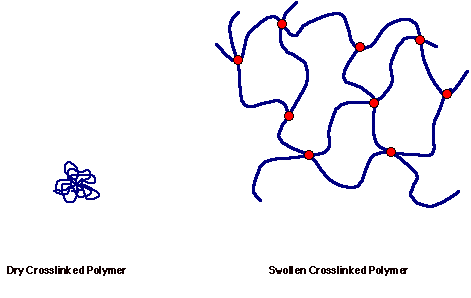
Figure 4. Comparison of a Dry Crosslinked Polymer with a Crosslinked Polymer Swollen in Solvent
PAC is nontoxic and environmentally benign, but it is not biodegradable. Because it is widely used for many applications, it poses an environmental problem from a landfill perspective. When PAC is used as an antiscalant or a dispersant, it becomes part of the wastewater. PAC is nonvolatile and not biodegradable, so the only way to remove it from the water is to precipitate it as an insoluble sludge. The sludge must then be landfilled1.
Thermally Synthesized Polyaspartates; Green Chemistry
The Donlar Corporation is a small company founded in 1990 that is committed to producing environmentally friendlier products. The Donlar Corporation developed an economic way to produce thermal polyaspartate (TPA) in high yield and with little or no waste products. Polyaspartate is a biopolymer synthesized from L-aspartic acid, a natural amino acid. Due in part to the carboxylate groups, polyaspartate has similar properties to the polyacrylates. Thus TPA can be used as a dispersant, an antiscalant, or a superabsorber, but it also has the added benefit of being biodegradeable.1 Biodegradation results in decomposition of TPA to environmentally benign products such as carbon dioxide and water. This negates the necessity for both the removal of TPA during sewage treatment and its disposal in a landfill.
In the process developed by The Donlar Corporation (Scheme 2),7 TPA is made by first heating aspartic acid to temperatures above 180 °C to produce polysuccinimide. This reaction is a step growth polymerization with water eliminated as the only byproduct. Using a catalyst, lower temperatures and shorter reaction times are possible. The yield of the polysuccinimide is ~ 97%.
The second step of the process utilizes aqueous sodium hydroxide at 60 °C to ring open the polysuccinimide to polyaspartate. Because the ring can open in two possible ways, two polymer linkages are observed, an a-linkage and a b-linkage.
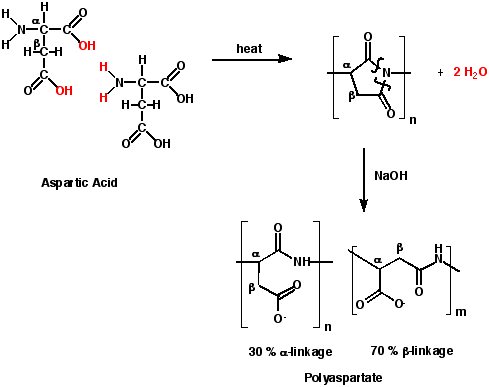
Scheme 2. Synthesis of Thermal Polyaspartate
Thermal Polyaspartate--Green Chemistry in ACTION
In April 1997, Donlar opened the world's largest manufacturing facility for biodegradable polyaspartates, in Peru, Illinois. The opening of this facility resulted in commercial availability of TPA. TPA is marketed and sold as a corrosion and scale inhibitor, a dispersing agent, a waste water additive, a superabsorber, and also as an agricultural polymer. As an agricultural polymer, TPA is used to enhance fertilizer uptake by plants. Not only does this mean less fertilizer is added to the soil, but it also reduces the environmental impact from fertilizer run-off.
The Donlar Corporation received the first Presidential Green Chemistry Challenge Award in the small business category in 1996. Donlar has received several U.S. and foreign patents for the manufacture, composition and end use of their bioenvironmental technology.
Questions
1. Show the reactions that occur when acrylic acid is crosslinked with trimethylolpropanetriacrylate using a free radical initiator.
2. In the reaction of acrylic acid and trimethylolpropanetriacrylate, how is the degree of crosslinking controlled?
3. In the ring opening of polysuccinimide to polyaspartate, the b-linkage is the preferred product. Why?
4. Polyanions such as PAC will expand in dilute aqueous solution so that the polymer coils are greatly expanded by the presence of charged groups. Predict what will happen to the polymer coils as the polyelectrolyte concentration increases. This is called the "polyelectrolyte effect".
5. Predict the swelling behavior of PAC in water versus an aqueous solution of NaCl.
6. Compare and contrast the polymer molecular weight in the initial and final stages of step growth polymerization with chain polymerization.
7. Polymeric antiscalants are generally low molecular weight polymers, whereas polymeric dispersants consist of higher molecular weight fractions. In a free radical reaction, what are the controlling factors in the molecular weight of the product?
References
1. Low, K.C., Atencio, A.M., Meah, A. R., Anderson, D.E., Batzel, D.A., Vallino, B., Rico, B., Ross, R.J., Harms, D., Spurrier, E., Below, F. "Production and Use of Thermal Polyaspartate Polymers", a proposal submitted to the Presidential Green Chemistry Challenge Awards program, 1996.
2. Wood, A. "Acrylics: Versatile Chemistry Adapts to Growth Market Emulsions and Superabsorbents Take the Lead", Chemical Week,1994, (Dec 22), 22.
3. Hater, W. "Environmental Scale Inhibitor for the Mining Industry", Corrosion98, 1998, 213.
4. Commercial poly(aspartic acid) and Its Uses; Low, K. C., Wheeler, A.P., Koskan, L.P. Advances in Chemistry Series 248; American Chemiscal Society: Washington, D.C., 1996.
5. Raloff, J. "EPA Honors a Greening of U.S. Industry", Science News, 1996, 13, 22.
6. Bucholz, F.L. "Superabsorbent Polymers", J. Chem. Ed., 1996, 73, 512.
7. Wheeler, A.P., Koskan, L.P. "Large Scale Thermally Synthesized Polyaspartate as a Substitute in Polymer Applications", Mat. Res. Soc. Symp. Proc., 1993, 292, 277.
Return to Green Module for Polymer Chemistry Home
