Green Chemistry
Greening Across the Chemistry Curriculum English | Versión en Español  | Versão em Português (Brasil)
| Versão em Português (Brasil) 
A Green Chemistry Module
Suggested Use: A descriptive inorganic course during a discussion of the production of paper, or an advanced inorganic course describing oxidizing agents or the effect of substituents on organometallic redox reactions.
TAMLTM Oxidant Activators: Green Bleaching Agents for Paper Manufacturing
David E. Marx, Chemistry Department, University of Scranton, Scranton, PA 18510
david.marx@scranton.edu
Background
For over 2000 years the making of paper has had a significant influence in the development of civilization. Worldwide consumption of paper now exceeds 300 million tons per year,1 with significant increases expected in the foreseeable future. It is estimated that there are 10,000 paper and paperboard mills in operation worldwide. Not surprisingly, per captia consumption of paper products is highest in the United States, exceeding 700 lbs per person each year.2 With 500 mills in operation, the U.S. is also the leading producer of paper, producing over 87 million metric tons each year. U.S. production represented over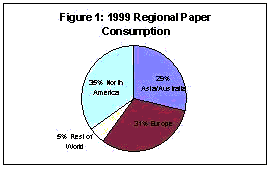 one third of the 1999 worldwide production a shown in Figure 1. Each year, the U.S. consumes more than 90 million metric tons of paper and paperboard, with a majority used to produce some 2 billion books, 350 million magazines, and 24 billion newspapers. In 1999, the growth of paper used in the communication industry (newsprint and magazines) grew by more than 5% over the previous year. While paper used in the communication industry has grown, the percentage of paper used for newsprint has experienced a decrease, falling from 33.5% of the total in 1990 to 28.9% presently. During the same time period, print and stationery use has seen an increase from 66.5% to 71.1%, with the largest increases in paper consumption coming from commerce firms employing direct marketing strategies. The use of paper has surged in every major category during the past year, including office printers and copiers, envelopes, and marketing flyers. Significant growth in consumption during the present decade is expected in developing countries in Africa and Latin America, with highest growth rates expected in Southeast Asia. During the 90's, the global paper communication market saw an increase 22.3% to 27.6% in the Asia/Australia/Fiji/New Zealand market. Asia recorded the fastest growth with a 10.0% increase in 1999.
one third of the 1999 worldwide production a shown in Figure 1. Each year, the U.S. consumes more than 90 million metric tons of paper and paperboard, with a majority used to produce some 2 billion books, 350 million magazines, and 24 billion newspapers. In 1999, the growth of paper used in the communication industry (newsprint and magazines) grew by more than 5% over the previous year. While paper used in the communication industry has grown, the percentage of paper used for newsprint has experienced a decrease, falling from 33.5% of the total in 1990 to 28.9% presently. During the same time period, print and stationery use has seen an increase from 66.5% to 71.1%, with the largest increases in paper consumption coming from commerce firms employing direct marketing strategies. The use of paper has surged in every major category during the past year, including office printers and copiers, envelopes, and marketing flyers. Significant growth in consumption during the present decade is expected in developing countries in Africa and Latin America, with highest growth rates expected in Southeast Asia. During the 90's, the global paper communication market saw an increase 22.3% to 27.6% in the Asia/Australia/Fiji/New Zealand market. Asia recorded the fastest growth with a 10.0% increase in 1999.
Of course, any changes in the paper industry dramatically effect the chemical industry as well. In the US and Canada alone, over 80 billion $US are used each year for the production of softwood bleached pulp. One American job in ten is related to the use of paper. These jobs include production, distribution, manufacture of paper based products and services, mailing, printing, publishing, and packaging. The combined total of these industries represents over $833 billion of economic activity each year.3 As impressive as this number is, one must realize that the figure is offset by the actual environmental and potential environmental damage resulting from traditional bleaching and other processes involved in the production of paper.
While the exact year is subject to debate, most experts agree that paper making had it start in China in 200 B.C.E. This art form was kept secret for almost 1,000 years until the process spread outside Asia. Paper making reached England in 1494, and America in 1690, with the building of the first U.S. paper mill by William Rittenhouse in Germantown, PA. Click on this link from the Institute of Paper Science and Technology for more information. Rittenhouse's method first used old rags in the process, but demand for paper eventually outgrew what could be supplied by rags. In the 1860's, U.S. paper mills turned to wood for the production of paper.
Most paper in the U.S. is made from the natural fibers in wood called cellulose. Both hardwoods and softwoods can be used in the paper making process. Fibers from softwoods, such as pine, tend to be over twice as long as hardwood fibers. Since longer fibers lend strength to the final paper product, softwood fibers are used in papers for grocery bags and boxes. Short fibers tend to make the final paper smoother. Fibers from hardwoods, such as oak, are blended with longer fibers to make printing and writing paper strong yet smooth. Hardwoods are also more dense than softwoods, resulting in greater fiber content per cubic foot of wood.
In countries without an abundance of trees, plants can be utilized in the paper making industry. In India, eucalyptus, straw, cotton, kenaf, and bamboo serve as alternate sources of fiber. Another important alternate source of fiber comes from recycled paper. In the U.S., 45% of the paper we use is recovered for use in the paper making industry, saving the need to utilize more trees in the production process. However, recycling will never eliminate the need for trees in the paper making process. In addition to contamination, cellulose fibers can only be recycled 5-7 times before they become too short and weak to be used. These fibers are washed away in the pulping process.
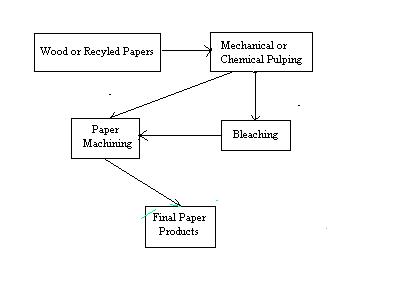
Figure 2: A General Schematic of the Paper making Process
A schematic diagram of the paper making process is shown in Figure 2. In the first step of the process, wood chips or recycled paper are broken down into individual fibers in a process referred to as pulping. Pulping can take place in one of two ways: mechanical or chemical. In mechanical pulping, the fibers are separated by grinding the wood. While this process is very efficient, paper made from mechanically pulped fibers tends to be weak, and discolor easily when exposed to light.4 This is due to the presence of residual lignin, a major component of wood which acts to bind cellulose fibers together. A generalized structure of lignin is shown in Figure 3.
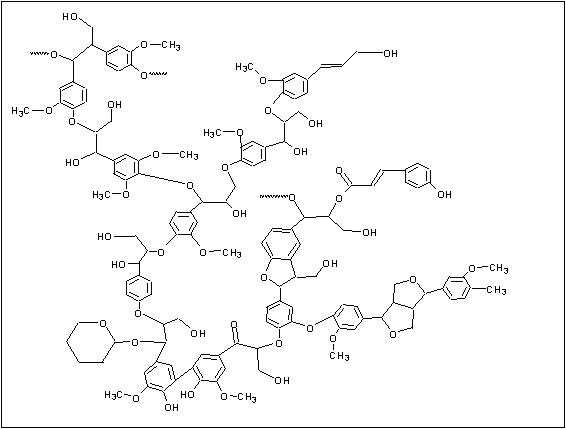
Figure 3: A Generalized Structure of Lignin (reproduced with permission from "Real-World Cases in Green Chemistry," copyright 2000 the American Chemical Society)
In chemical pulping, wood chips or recycled paper are combined with water and chemicals and heated until the cellulose fibers separate. The fibers are then subjected to a bleaching process.4,5 The primary reason for the bleaching of pulp is to remove the lignin content since it causes a brown discoloration in the final paper. Removal of the lignin, which is a chemically complex material, produces a lighter shaded pulpwood. In addition to cellulose, lignin and hemicellulose are also distributed throughout the cell walls. The relative proportions of these three components vary, but in typical softwoods, the relative proportions are 28.0%(w/w) lignin, 28.7 %(w/w) hemicellulose, 40.3%(w/w) cellulose, and an additional 3% "extractables". These extractables include fats and esters, phenolic materials and tannins, and terpenes and resin acids which can be removed with organic solvents. Trace quantities of inorganics are present as heavy metals. In the pulping process, lignin is degraded into chromophoric (color producing) groups which otherwise would account for 90% of the dark color characteristic of unbleached pulp.
Conventional bleaching of pulp often takes place by the Kraft Process. This consists of a series of chemical processing steps using alkali, acid, hydrogen and sodium peroxide, oxygen, dithionite salts, sodium bisulfite, and wash water processes followed by chlorinating treatments to remove any residual lignin. The chlorinating treatments make use of either chlorine gas, hypochlorite salts and/or chlorine dioxide. Unfortunately, while this method produces bright white paper, it also causes environmental problems through the production and release of organochlorine compounds. These compounds are formed by the reaction or of organics present in the pulp with the chlorine-containing oxidizing agents. The reaction of chlorine and lignin produces chlorinated aromatic rings. Among the organochlorines that are produced are 2,3,6,7-tetrachlorodibenzo-4-dioxin (TCDD, also known by the generic term dioxin) and furans.4,5 TCDD is the most common and most toxic dioxin produced in paper manufacturing.6
While an exact relationship between TCDD and human health risks has not been formally established, the U.S. Environmental Protection Agency (EPA) has noted possible effects of TCDD, namely chloracne, behavioral effects and learning disorders, decreased male sex hormone, diabetes, immune system toxicity, and increased cancer risk at elevated dioxin concentrations in the body. TCDD has also been demonstrated to have a tetratogenic effect. TCDD is over 10,000 times more acutely toxic than the cyanide ion. When a person is exposed for long periods of time, cirrhosis of the liver and damage to kidneys, spleen, central nervous system, lungs and pancreas may occur. Memory and concentration disturbances have also been reported. It should be noted that the EPA has stated that the average U.S. citizen has no particular exposure to TCDD besides what is routinely eaten in food. It is estimated that 95% of our dioxin exposure is through food intake, mainly through the consumption of red meat, fish and dairy products. Because of the nonpolar nature of TCDD, like many organochlorines, it is far more soluble in the fatty tissues of animals than in water. Thus, once ingested, these compounds tend to bioaccumulate rather than be excreted by the body. In the food chain this leads to higher concentrations in higher species, a process known as biomagnification. The synergy between bioaccumulation and biomagnification has been shown to lead to TCDD contaminant levels in fish up to100,000 times that found in the native surroundings.7
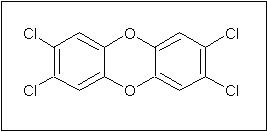
Figure 4: Structure of 2,3,6,7-tetrachlorodibenzo-4-dioxin
In the next stage of paper making following pulping, fillers and special additives are combined with the pulp to give the paper special characteristics, such as gloss, absorbency, or water resistance. The resultant slurry is sprayed from a vat, referred to as a headbox, onto a plastic screen which moves very quickly through the paper machine. As the screen carries the pulp mixture along, water drains out and the fibers bind together. The web of paper is pressed between a series of felt-covered, heated rollers to make a smooth surface. At this point, the dried paper is slit into narrower rolls or sheets, and removed from the paper machine.
The chemical composition of the effluents of pulp and paper mills utilizing elemental chlorine is exceedingly complex. Approximately 4 kg of organically bound chlorine is produced for every one ton of pulp. Over 250 compounds have been identified; 180 of which are chlorinated. Several classes of chlorinated compounds have been identified, including chlorophenols, catechols, guaiacols, and syringols. Chlorinated phenols are known to be the pre-cursors of polychlorinated dibenzo-p-dioxins (PCDDs or dioxins) and dibenzofurans (PCDFs or furans). For this reason the effluent of pulp and paper plants is considered to be highly toxic. A study of North American pulp plants indicated the presence of chlorinated dioxins in bleached pulp samples at 1-51 parts per trillion (ppt), in primary sludge at 3.3-180ppt, and in the final effluent at 3-120 parts per quadrillion.
As a result of the concern over dioxins, and organochlorines in general, the EPA has banned the use of elemental chlorine in the bleaching of paper by April 2001. Current estimates indicate that up to 20% of the world's bleached paper is produced with elemental chlorine. Another 54% of bleached paper is now produced with chlorine dioxide, dramatically lowering the dioxin and furan emission by >90%.8 This process is referred to as ECF, or Elementary Chlorine Free paper. The remainder of the bleached paper, a small fraction, is produced using chlorine-free alternates. This process is referred to as TCF, or Totally Chlorine Free. Paper producers tend to shy away from this latter method due to low production efficiencies; about 10% more wood has to be used, and the fiber length is shortened. Thus the recyclability of the paper is reduced.
GREEN CHEISTRY: TAMLTM
Work performed in the laboratories of Terrence Collins at Carnegie Mellon University has produced a series of oxidant activators referred to as "tetraamido-macrocyclic ligand activators," abbreviated as TAML.TM For this work, Collins was awarded aPresidential Green Chemistry Challenge Award in 1999. These activators catalyze hydrogen peroxide oxidation reactions in the paper bleaching process.9 Hydrogen peroxide oxidation is considered to be a TCF, environmentally friendly process. This is because only water and oxygen are formed as products when hydrogen peroxide is consumed. This is in sharp contrast to the dioxins and furans produced through the use of elemental chlorine as a bleaching agent. Furthermore, these activators are free of toxic functional groups and contain no elements found to be harmful to life. As an added benefit, TAMLTM has been shown to work at ambient temperatures and pressures, providing the paper industry with the first low-temperature peroxide delignification process. This leads to significant savings in energy costs and environmental benefits such as the decreased consumption of nonrenewable resources (fuels) with a concomitant reduction in greenhouse gas production.
TAMLTM activators are based on iron macrocyclic structures as shown:
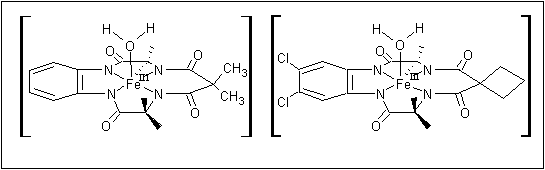
Figure 5: TAMLTM Activators (reproduced with permission from "Real-World Cases in Green Chemistry," copyright 2000 the American Chemical Society)
Many metals have shown the ability to improve the oxidizing ability of hydrogen peroxide. The most common metal employed for these purpose is iron. The reactivity of this type of system was observed in 1894 by Fenton, but the utility of these reactions wasn't recognized until the 1930's when the mechanism was elucidated. Fenton's reagent is a stronger oxidizing agent than H2O2 by itself. It is capable of oxidizing dihydrogen and organic substrates that often resists oxidation. It has also been used to initiate polymerization reactions. The overall reaction is:10
2Fe2+ (aq) + H2O2 + 2H+ ![]() 2Fe3+ (aq) + 2H2O
2Fe3+ (aq) + 2H2O
This reaction, however, does not explain the increased oxidizing ability of this system. It is now known that the species responsible for the increased oxidative powers is the highly reactive hydroxy radical (.OH) which forms when ferrous or ferric ion interacts with peroxide in aqueous solution according to the following scheme:
Fe3+ + H2O2![]() Fe2+ + -OH + OH
Fe2+ + -OH + OH
OH + H2O2![]() HOH + HO2
HOH + HO2
HO2 + HOOH ![]() HOH + O2 + OH
HOH + O2 + OH
Fe3+ + H2O2![]() Fe2+ + HO2 + H+
Fe2+ + HO2 + H+
Fe3+ + HO2![]() Fe2+ + O2 + H+
Fe2+ + O2 + H+
Fe3+ + OH ![]() FeOH2+
FeOH2+
OH + S ![]() P
P
In the final step substrate S is oxidized to form product P.
It is interesting to note that the TAMLTM system proposed by Collins operates as a "Non-Fenton's" based reaction. The hydroxyl radical produced in a Fenton's-type reaction would be highly reactive and would tend to scavange any organic matter, rendering it too indiscriminate for selective oxidation. Instead, Collins has proposed the formation of a metal-oxo reactive intermediate formed from the abstraction of an oxygen atom of hydrogen peroxide by the metal ion.11, 12 As a result, water would be formed along with a two electron oxidized metal-oxo species. Much of Collins work has focused on the design of multidentate ligands that tend to release electrons and stabilize the metal-oxo species. In addition, the ligands have been designed to be resistant to oxidation for prolonged periods in order for a catalytic oxidation cycle to be employed. Otherwise, the ligand would degrade, followed by release of the metal center, followed by traditional Fenton's-type reaction chemistry.
Collins, et al. have recently reported that the TAMLTM complex catalyzes hydrogen peroxide oxidation of pulps. The reaction was shown to proceed rapidly at temperatures ranging from ambient to 90oC, and resulted in pulps with the kappa number reduced from 21.5 to 7.8 in 60 minutes.13 The kappa number refers to the amount of lignin left in the pulp, thus in the experiment just outlined the lignin was reduced to one third of the original amount. When chelating agents were added to remove contaminant metal ions which were prematurely decomposing the hydrogen peroxide, the delignification proceeded more rapidly than in the control experiment. Additionally, the removal of lignin was greater at 50oC than the control at 90oC. Under similar conditions, TAMLTMactivated hydrogen peroxide destroyed trichlorophenol, a pre-cursor to TCDD formation.
The activator lifetimes have undergone much study by the Collins group. Initially the ligands employed in the TAMLTM complexes lasted only minutes before they were oxidized. At present, they survive on the order of hours. Ligands designed to last days are currently being designed and studied. This has led to the development of adjustable activator lifetimes, allowing one to be chosen for an intended purpose. Clearly, this is an environmentally important feature.
Collins, et al. have developed a series of ligand protection rules as a guide to designing stable "metalloredox-active oxidants."
These oxidants will oxidize a substrate without a being accompanied by a formal oxidation change of the metal center. Instead, the metal activates the reactive species and places the oxidant and substrate in a geometrically favorable arrangement to allow the oxidation reaction to take place. The four rules that Collins has developed are12:
"I. 'For chelate rings, a hydrogen atom should not be placed on an atom that is b to an oxidizing metal center, if the a-atom can support an increase in the bond order with the b-atom.'
II. 'A heteroatom should not be attached to a five-membered chelated ring on an atom that is g to an oxidizing metal center, if the heteroatom has an available lone pair to stabilize forming cationic character on the g-atom as the endocyclic b-g bond is oxidatively cleaved by the metal.'
III. 'A heteroatom should not be employed as an a-donor atom in a five-membered chelate ring, if it has an available lone pair to stabilize forming cationic character on the b-atom as the endocyclic b-g bond is oxidatively cleaved by the metal.'
IV. 'If the goal is to produce a strong electron transfer oxidant, amido-N donors should be avoided as internal ligands in acyclic chelate ligands.' "12
These four rules lead to the use of macrocyclic tetraamides as strong oxidizing agents.
Adoption of TAMLTM based technology has the potential of resulting in significant savings if employed at 100% usage in the paper and pulp mills in the U.S. Energy savings alone from the lower water temperatures employed with TAMLTM would result in the savings of 38.9 x 1012 BTU's per year, or the energy equivalent of 23.15 million tons of coal.11 In addition, it is estimated that billions of dollars could be saved each year on money that would otherwise be spent on pollution abatement and related equipment designated to reduce the emission of chlorinated compounds formed by traditional bleaching methods.
It is interesting to note that under the present definitions, a paper plant employing 100% TAMLTM based technology would still not be considered to be operating as a TCF plant. This is due to the fact that recycled papers are used as a fiber source and the nature of these papers remains uncharacterized. In order to be considered a true TCF plant, 100% virgin pulp would have to be utilized. This has led to several manufacturing organizations recommending the use of PCF (Processed Chlorine Free) paper over TCF paper.14
The TAMLTM activator technology is patented and licensing agreements for commercialization have been issued. Other uses such as laundry applications (to reduce the problem of dye transfer) are being considered. In addition, these complexes are being proposed for use in the purification of drinking water,9 and for use as a dye inhibition agent. The complexes have been shown to oxidize loose dye molecules that have been rinsed from fabrics in washing machines.9 TAMLTM oxidizes these dye molecules before they have the opportunity to adhere to other fabrics in the wash cycle. An excellent on-line slide presentation featuring Professor Collins addressing some of the chemistry described here can be viewed by clicking here.
Questions
1. What are some advantages of using the TAMLTM/hydrogen peroxide bleaching system over chlorine systems?
2. What is the difference between PCF and TCF paper? (see the Association of Vermont Recyclers)
3. What is a metallo-redoxactive oxidant?
4. What metals other than iron would you propose for you in this type of system? Why?
5. Why is a macrocyclic tetraamide ligand a good choice over other ligands for this reaction?
6. What would be the by-product of the hydrogen peroxide decomposition in the delignification process?
7. How do lignin's aromatic rings become chlorinated in the presence of chlorine gas? Show a mechanism.
8. What other macrocyclic ligands would you suggest studying for their potential to catalyze hydrogen peroxide delignification
reactions?
9. When considering only the donor atoms of the tetraamide ligands, along with the iron center and coordinated water
molecule as shown in Figure 5, what is the point group of the complex?
10. Where do you commonly encounter Kraft paper in your life. Cite some examples over the last week.
Fun Facts
Did you know that:
1 cord of wood has the dimensions of 8 feet wide by 4 feet deep by 4 feet high? From this cord of would we could obtain approximately one of the following groups: 7,5000,000 toothpicks
1000-2000 lbs of paper (depending on the process)
61,370 standard envelopes
4,384,000 postage stamps
30 Boston rockers
Ben Franklin was the first paper merchant in America?
The US recovers more paper than it landfills each year?
References
1. See https://www.fs.fed.us/pnw/sev/rpa/ince_files/sld007.htm for consumption and monetary statistics of the paper and pulp
industry in the US.
2. See https://www.tappi.org/paperu/all_about_paper/paperClips.htmfor a variety of information about paper and paper
making.
3. For an excellent comprehensive review, see "Towards Zero-Effluent Pulp and Paper Production: The Pivotal Tole of
Totally Chlorine Free Bleaching," Johnston, P.A., et al., Greenpeace Research Laboratories, Technical Report 7/96,
Exeter, UK which can be viewed and downloaded at: https://xs2.greenpeace.org/~toxics/reports/tcf/tcf.html
4. Weinstock, Ira A., et al., "A New Environmentally Benign Technology for Transforming Wood into Paper: Engineering
Polyoxometalates as Catalysts for Mutiple Processes, J. Mol. Catal., A: Chem, 1997, 116, 59-84
5. Chinier, Philip J., Survey of Industrial Chemistry, Wiley-VCH: New York, 1992
6. Baird, Colin, Environmental Chemistry, 2nd ed., W. H. Freeman: New York, 1999
7. See Dioxin Homepage at: www.enviroweb.org/issues/dioxin/index.html
8. Hume, Claudi, Adams, Jarret, "Weak Market Slows Migration from Chlorine," Chemweek, 1999, 17, 46-48
9. Terrence J. Collins, TAML Activators: General Activation of Hydrogen Peroxide for Green Oxidation Processes, a
proposal submitted to the Presidential Green Chemistry Challenge Awards Program, 1999
10. D. Katakis and Gordan, G., Mechanisms of Inorganic Reactions, Wiley: New York, 1987, pg 6
11. Collins, T. J., Gordon-Wylie, S. W., J. Amer. Chem. Soc., 1989, 111, 4511-4513
12. Collins, T. J., J. Amer. Chem. Soc., 1994, 27, 9, 279-285
13. Collins, T. J., et al., Development of the PFe Process: A New Catalyzed Hydrogen Peroxide Bleaching Process.,
Proceedings of the 53rd APPITA Annual Conference, APril 1999, Rotorua, New Zealand
14. See the Association of Vermont Recyclers at: https://www.sover.net/~recycle/free.html
