Particles in the atmosphere (salt and ice crystals are examples) have been shown to affect the photochemical properties of the species that adhere to them. Depending on the molecule adsorbed, and the substrate onto which it is adsorbed, the efficiency of photochemistry may be augmented or attenuated by adsorption. The members of this group are investigating the effects of the local environment on the photochemistry of small molecules. Our original studies focused on sublimated films of alkali halides, maintained at cryogenic temperatures and used as substrates. Subsequent studies have dealt with molecules trapped in cryogenic solids. The samples are characterized via their infrared spectrum. The system is then irradiated at a variety of temperature/coverage conditions, utilizing LASER and arc lamp sources.
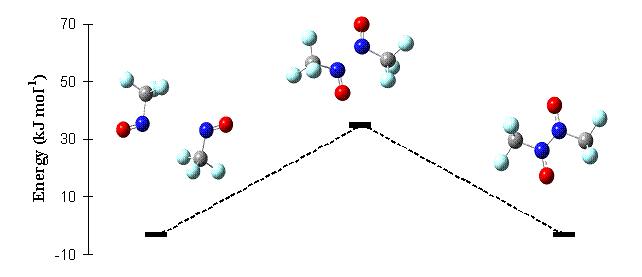
We have found evidence of surface-induced quenching in the CF3NO-alkali halide systems. We attribute this to a removal of excess vibrational energy from the molecule (by surface and bulk phonons of the halide) before that excess energy leads to fragmentation of the parent molecule. Placing spacer layers (~0.1 micrometer in thickness) of condensed inert gases between the adsorbate and alkali halide reduces the quenching, leading to the production of a photodimer of the parent compound. The photodimer is also formed in the rare gas matrices.

Experiments involving the ultraviolet photochemistry of
matrix-isolated biacetyl (C4H6O2) have led to the characterization of a
new pathway (the ketene channel) that results from a triplet-triplet
transition in the parent compound. Studies involving matrix-isolated complexes with water are also being carried out.
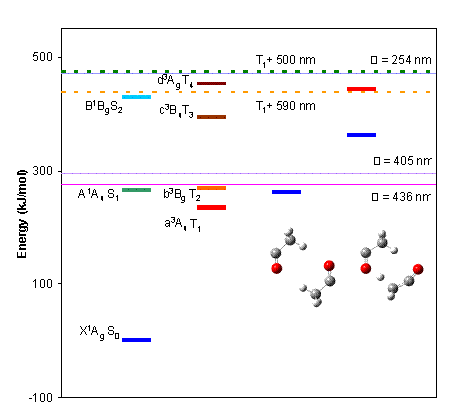
All members of the group are using computational methods (primarily density functional theory with Gaussian) to elucidate the photochemical pathways observed in their experiments.

Raymond Diorio
Vinh Nguyen